Two weeks ago, I wrote about how children’s hospitals were being overwhelmed by an early and severe onset of the winter respiratory virus season, with respiratory syncytial virus (RSV) infections being particularly problematic. Since then, the problem has continued with many regions of the country still seeing record numbers of hospitalizations and extreme difficulty finding inpatient and intensive care beds for children in need of them. States are declaring emergencies and the AAP has asked the Biden administration to do so federally, though in keeping with the historical response to pediatric healthcare issues they have declined to do so.
On a personal note, the Boston area has seen some improvement over the past week or so and we all are very hopeful that this is not a fluke. We are still busy, but there is breathing room in regards to getting kids from the ED to the inpatient unit, and we have put on a hold on our surge staffing plan…for now. PICU beds are holding at around 90% full in the region as well. Unfortunately, cases of influenza are now rapidly on the rise so it’s unclear how long this much-needed breather will last.
You may be wondering why RSV is so bad this season. You may have even heard the phrase “immunity debt” tossed around. Many people are blaming our response to the SARS-CoV-2 pandemic, claiming that preventing children from getting the usual viral infections for a couple years has resulted in a lazy and less effective immune system. While there is some truth to the association between pandemic control measures and a worse than typical RSV season, the kids’ immune systems are alright and so-called “immunity debt” is nonsense.
But before I explain why this RSV season is hitting children so hard, a brief primer on the disease that practically defines pediatric inpatient medical training and practice.
What is RSV?
RSV is a single-stranded, negative-sense RNA virus that targets the respiratory tract in humans and causes seasonal outbreaks all over the world. In the United States, RSV season typically runs from October to May with a peak around January or February. Clearly, as the past three years have shown us, this seasonal pattern can be impacted by behavioral factors such as school closures, masks, and distancing. We saw spring and summer spikes of RSV in 2021, for example, when people cut back on these measures.
Virtually every child catches RSV by the age of 2 years. This age group, particularly the very young infants, tends to have the most severe disease because it is usually a child’s first RSV infection and they have smaller airways. Long-lasting protection from additional infection doesn’t occur after these initial cases, and we are potentially reinfected year after year. RSV is a common cause of colds in older children and adults, and again becomes a serious health concern in the elderly, or never stops being one in people of any age with certain immune deficiencies or underlying respiratory, cardiac, or neurologic disease.
In these younger children, RSV is the most common cause of lower respiratory tract infections and the most likely reason for them to be hospitalized. Hospitalization with RSV has been much more common than I bet most people realize, even before this season’s historic rates. Globally, roughly 4 out of every 1,000 children under 5 years become ill enough to need a hospital bed. If you focus on the highest risk groups, it gets much worse with 20 per 1,000 children less than 6 months and 60 per 1,000 premature infants being hospitalized every year.
Hospitalization is less likely in the United States, where the general nutritional status of children is more stable than in developing regions of Africa and Asia, for example, but it is still common. Here roughly 3 per 1,000 kids under 5 years are hospitalized. In our highest risk populations of infants less than 6 months, roughly 15 per 1,000 need an inpatient or PICU bed.
Death from RSV is uncommon in the United States, but this isn’t the case everywhere. RSV is to blame for about 2% of deaths in the first month of life worldwide, and 7% from months 2 through 12, making it second only to malaria in that age range. Most of these deaths occur in term infants, largely because premature infants tend to die from prematurity-related complications in resource-limited regions.
In the United States, deaths from RSV still occur, typically in premature infants or young children with immune deficiencies or underlying heart disease. As I mentioned earlier, RSV also causes problems across the lifespan in certain high-risk groups, but I’m a pediatrician and am keeping this focused on kids. Sorry.
What do RSV infections do?
Before RSV starts wreaking havoc in the upper and lower airways of babies and grandparents all over the world, it has to gain access to them first. Usually the virus is spread by secretions or surfaces full of or covered in the virus, meaning direct contact is the most likely way someone catches RSV. The virus can remain viable for several hours on surfaces, including our unwashed hands. Large droplets of nasty RSV-containing mucous likely also help spread RSV, which is why in the hospital I wear a gown, gloves, and a mask in patient rooms.
Infants who become ill with RSV usually acquire it from an older sibling, who may or may not even be symptomatic. The virus can be shed in mucous secretions for days to weeks, though it tends to peak in the first week of illness, which is why measures such as hand washing and distancing during RSV season play such an important role in reducing spread. Add in masks and you can see how we had a season like 2020-2021 with virtually no RSV activity in the United States.
So before making these little ones sick, RSV has to gain access to their ocular or nasopharyngeal mucous membranes. Once there, it has to get past the immune system. Sadly, it is very good at doing just that.
I mentioned above that essentially every child catches RSV in the first couple years of existence, and also that we continue to catch it over and over again throughout our lives. This means that infection doesn’t result in protection against reinfection. But previous infection, and the subsequent development of antibodies against RSV, does reduce the severity of future infections even years later. Infants born to mothers with high levels of RSV antibodies are also less likely to have a severe infection. Keep that nugget handy for later on in the post because it’s important.
Once RSV gains access to our bodies, evades the immune system, and begins replicating in the nasal passages and throat, it can begin moving towards the lungs. And again, this is much more likely to happen in young and small children with their first RSV infection. The virus, having infected the lining of the airway, moves from cell to cell or is carried by the movement of secretions from the upper to the lower tract. Within 1 to 3 days of symptom onset, the smallest airways deep in the lungs, known as bronchioles, are involved. These bronchioles end in the tiny air sacs, the alveoli, where oxygen and carbon dioxide are exchanged.
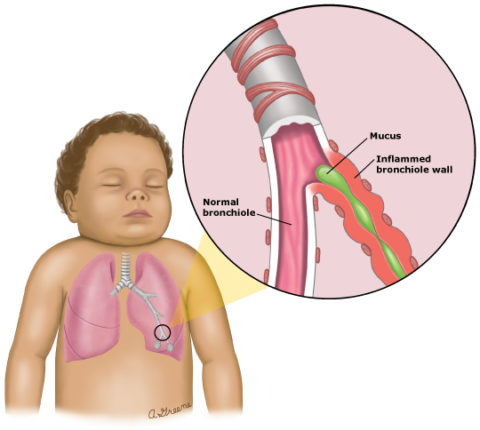
RSV causes necrosis of the cells lining the respiratory tract as well as an inflammatory response that results in airways full of nasty, thick gunk (non-medical term). These airways, from the nose down to the bronchioles, can become obstructed and this can make it very difficult for children to breath. It can also make it difficult for the lungs to perform gas exchange, which is kind of their point.
The name for the clinical presentation of RSV infections in these young children is bronchiolitis, meaning inflammation of the bronchioles, and there is really nothing else like it in pediatric medicine. It is such a classic pattern, in fact, that it can be diagnosed without any blood tests or imaging, just an old fashioned history and exam. RSV happens to be the most common cause of bronchiolitis by far, though several other viruses can do it as well. There is no way to clinically differentiate bronchiolitis caused by RSV from bronchiolitis caused by one of these other viruses, however, which is why testing can sometimes be helpful.
Children with bronchiolitis have copious amounts of nasal secretions that alone would make it hard for a small and potentially obligate nasal breathing infant to breathe and feed. They also tend to have significant cough that can be quite uncomfortable, and in general these kids are pretty miserable even when their work of breathing isn’t too bad. Children who require hospitalization sometimes just need help with maintaining hydration because of poor feeding, and/or supplemental oxygen to make up for impaired gas exchange in the alveoli. Many have true difficulty breathing though.
In order to move air in and out with thick secretions plugging their airways, young children with bronchiolitis breathe harder and faster. This often involves the use of accessory respiratory muscles of the chest and the development of retractions, where the skin can be seen sucking in above the collar bones, between the ribs, or under the sternum and ribs. In severe cases, children will have nasal flaring or even grunting at the end of expiration.
Grunting is an attempt by the child to provide a bit of positive end expiratory pressure for themselves at the end of each breath, which is known as PEEP when dialing it into the settings of a ventilator. In babies with bronchiolitis, however, it is an automatic process that helps to keep the alveoli at the end of the bronchioles from completely collapsing and is a last ditch effort to make the next inhalation a bit easier. Grunting in an infant is an ominous sign of impending respiratory failure.
If you lay a stethoscope on the chest of one of these babies, you might hear a range of abnormal sounds such as coarse breathing, crackles, or wheezing. Regardless of what you hear, you will almost certainly see a child who is breathing hard, fast, and uncomfortably. Some babies, mostly when premature and still only a few weeks old or less, may even have significant periods of time where they just stop breathing altogether without stimulation.
How do we treat RSV infections?
Unfortunately, there is no specific therapy that improves the outcomes of RSV. There is no antiviral, steroid, or breathing treatment that has ever been proven to make these kids get better faster. What we do in the hospital for the most part is provide supportive care, with fluids, tube feeds, oxygen, and nasal suctioning. A lot of these babies require higher levels of respiratory support that provides positive pressure into the airway via the use of a high-flow nasal cannula, CPAP, or even mechanical ventilation through an endotracheal tube. Thankfully, in the vast majority of cases RSV infections are self-limited and there is a full recovery.
Can we prevent RSV infections?
At this time, there aren’t any great options for preventing RSV infections. Infection control measures like handwashing, distancing, and masks are clearly helpful. There is also a monoclonal antibody (palivizumab) that is given to certain very high-risk populations of premature infants that is extremely expensive and must be dosed every month during RSV seasons. It lowers the risk of hospitalizations but has not been shown to reduce mortality.
Several RSV vaccine candidates are in the works, but one that was reported on just this month looks particularly promising. Pfizer has completed a phase 3 study involving more than 7,000 subjects that studied the effectiveness of a vaccine given to pregnant women at reducing the risk of severe RSV in infants. The results are impressive:
In a phase 3 study, the Pfizer vaccine was about 82% effective against severe illness from respiratory syncytial virus, or RSV, in the first 90 days of life in babies born to vaccinated women, according to the company. The vaccine was also about 70% effective against severe infections through the first six months of life.
The vaccine appears to be less effective at preventing any infection, however. Still, a reduction of any case of RSV in this high-risk population by 57% in the first 90 days and 51% in the first 6 months would be of huge benefit. The FDA will get a chance to review the data this year, and there is a good chance this vaccine will be available in time to make a difference during the 2023-2024 RSV season.
Why is this RSV season so awful?
Why is this season so awful? As I mentioned earlier, it isn’t because of lazy immune systems caused by overzealous COVID precautions. That’s just nonsense. But it is because of appropriate COVID precautions that saved thousands of lives during the pandemic so far.
When it comes to RSV, the first infection is usually the most severe and more likely to shed virus for a longer period of time. In a typical year prior to the pandemic, lots of babies got RSV for the first time and a certain percentage of them required hospitalization. But many babies who might have gotten infected by an older sibling didn’t because that big brother or sister had their first round of RSV during the previous season and either didn’t catch it again or had less severe disease.
Because of COVID precautions, many infants who would have gotten RSV in 2020 and 2021 did not. So the pool of children with no immunity whatsoever against the virus in late 2022 was dramatically larger than any previous year because it included young infants and those now 2-3 year old toddlers. Now that most people and schools have relaxed those COVID precautions, record numbers of children have become infected with RSV for the first time, and that certain percentage requiring hospitalization, though it hasn’t changed, is now out of a much larger number and has overwhelmed the system.
If I’m correct, this means that the next RSV season should be considerably milder than the current one. I wouldn’t be surprised if it is milder than the pre-pandemic average in severity. In fact, and I’m just spitballing here, it might take a few seasons to re-equilibrate, assuming that there isn’t a return to widespread masking and distancing.
Perhaps I’m being naïve, but it seems like a reasonable thing to do would be for schools and the general public to maybe consider the health of children and perhaps put some precautions in place during peak respiratory virus season that mitigates the severity to a meaningful degree. Does it have to be all or nothing when it comes to masks, for example? I still wear a mask when I’m in stores or on the subway because I don’t want to potentially catch and spread RSV to a high-risk child.
Conclusion: Wash those hands, keep some distance when possible, and mask up
That’s it really. RSV is bad. It’s a lot worse than usual this year because more kids are susceptible to catching it for the first time. It might be better next year but we should do things that help make it better now. You can catch and spread RSV, so wash your hands after potential exposures, be careful about getting close to young children (or the elderly or immune compromised) when you have any viral symptoms, and consider wearing a mask in high risk situations until things settle down.
